- Record: found
- Abstract: found
- Article: not found
Haploinsufficiency of the Mus81–Eme1 endonuclease activates the intra-S-phase and G 2/M checkpoints and promotes rereplication in human cells
Read this article at
Abstract
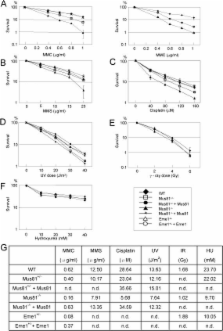
The Mus81–Eme1 complex is a structure-specific endonuclease that preferentially cleaves nicked Holliday junctions, 3′-flap structures and aberrant replication fork structures. Mus81 −/− mice have been shown to exhibit spontaneous chromosomal aberrations and, in one of two models, a predisposition to cancers. The molecular mechanisms underlying its role in chromosome integrity, however, are largely unknown. To clarify the role of Mus81 in human cells, we deleted the gene in the human colon cancer cell line HCT116 by gene targeting. Here we demonstrate that Mus81 confers resistance to DNA crosslinking agents and slight resistance to other DNA-damaging agents. Mus81 deficiency spontaneously promotes chromosome damage such as breaks and activates the intra-S-phase checkpoint through the ATM-Chk1/Chk2 pathways. Furthermore, Mus81 deficiency activates the G 2/M checkpoint through the ATM-Chk2 pathway and promotes DNA rereplication. Increased rereplication is reversed by the ectopic expression of Cdk1. Haploinsufficiency of Mus81 or Eme1 also causes similar phenotypes. These findings suggest that a complex network of the checkpoint pathways that respond to DNA double-strand breaks may participate in some of the phenotypes associated with Mus81 or Eme1 deficiency.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Linkage of ATM to cell cycle regulation by the Chk2 protein kinase.
- Record: found
- Abstract: found
- Article: not found
Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine.
- Record: found
- Abstract: found
- Article: not found

