- Record: found
- Abstract: found
- Article: found
β-Cell Replacement Strategies: The Increasing Need for a “β-Cell Dogma”
Read this article at
Abstract
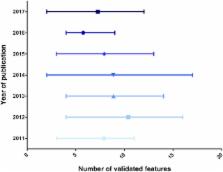
Type 1 diabetes is an auto-immune disease resulting in the loss of pancreatic β-cells and, consequently, in chronic hyperglycemia. Insulin supplementation allows diabetic patients to control their glycaemia quite efficiently, but treated patients still display an overall shortened life expectancy and an altered quality of life as compared to their healthy counterparts. In this context and due to the ever increasing number of diabetics, establishing alternative therapies has become a crucial research goal. Most current efforts therefore aim at generating fully functional insulin-secreting β-like cells using multiple approaches. In this review, we screened the literature published since 2011 and inventoried the selected markers used to characterize insulin-secreting cells generated by in vitro differentiation of stem/precursor cells or by means of in vivo transdifferentiation. By listing these features, we noted important discrepancies when comparing the different approaches for the initial characterization of insulin-producing cells as true β-cells. Considering the recent advances achieved in this field of research, the necessity to establish strict guidelines has become a subject of crucial importance, especially should one contemplate the next step, which is the transplantation of in vitro or ex vivo generated insulin-secreting cells in type 1 diabetic patients.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: not found
Generation of functional human pancreatic β cells in vitro.
- Record: found
- Abstract: found
- Article: not found
Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells.
- Record: found
- Abstract: found
- Article: not found