- Record: found
- Abstract: found
- Article: not found
IDENTIFICATION OF ERYTHROFERRONE AS AN ERYTHROID REGULATOR OF IRON METABOLISM
Read this article at
Abstract
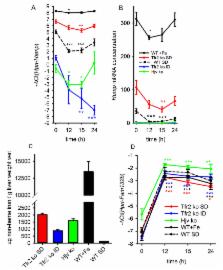
Recovery from blood loss requires a greatly enhanced supply of iron to support expanded erythropoiesis. After hemorrhage, suppression of the iron-regulatory hormone hepcidin allows increased iron absorption and mobilization from stores. We identified a new hormone, erythroferrone (ERFE), which mediates hepcidin suppression during stress erythropoiesis. ERFE is produced by erythroblasts in response to erythropoietin. ERFE-deficient mice fail to suppress hepcidin rapidly after hemorrhage and exhibit a delay in recovery from blood loss. ERFE expression is greatly increased in murine Hbb Th3/+ thalassemia intermedia where it contributes to the suppression of hepcidin and systemic iron overload characteristic of this disease.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism.
- Record: found
- Abstract: found
- Article: not found
Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs).
- Record: found
- Abstract: found
- Article: not found
