- Record: found
- Abstract: found
- Article: found
High-Yield Methods for Accurate Two-Alternative Visual Psychophysics in Head-Fixed Mice
Read this article at
Summary
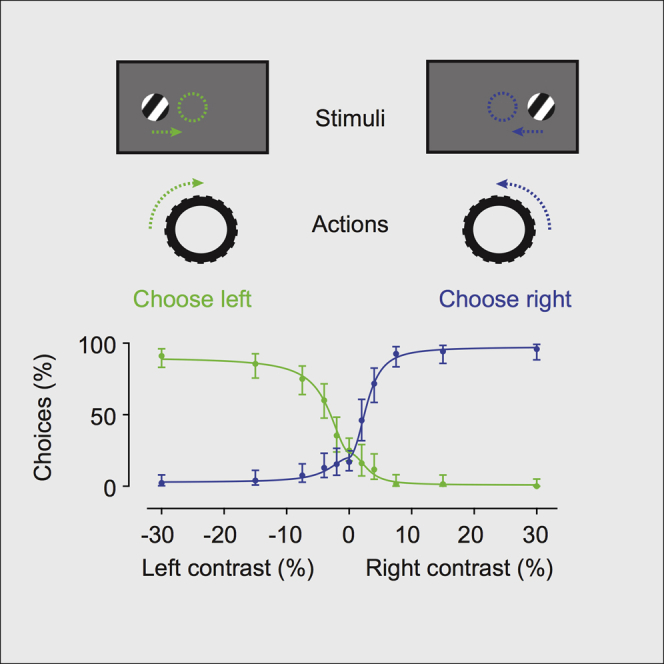
Research in neuroscience increasingly relies on the mouse, a mammalian species that affords unparalleled genetic tractability and brain atlases. Here, we introduce high-yield methods for probing mouse visual decisions. Mice are head-fixed, facilitating repeatable visual stimulation, eye tracking, and brain access. They turn a steering wheel to make two alternative choices, forced or unforced. Learning is rapid thanks to intuitive coupling of stimuli to wheel position. The mouse decisions deliver high-quality psychometric curves for detection and discrimination and conform to the predictions of a simple probabilistic observer model. The task is readily paired with two-photon imaging of cortical activity. Optogenetic inactivation reveals that the task requires mice to use their visual cortex. Mice are motivated to perform the task by fluid reward or optogenetic stimulation of dopamine neurons. This stimulation elicits a larger number of trials and faster learning. These methods provide a platform to accurately probe mouse vision and its neural basis.
Graphical Abstract
Highlights
-
•
A platform to probe visual discrimination and its neural basis in head-fixed mice
-
•
Extension of classic two-alternative choice design to unforced choices
-
•
Probabilistic observer model for mouse decisions and effects of inactivation
-
•
Optogenetic stimulation of dopaminergic neurons can replace water control
Abstract
Burgess et al. introduce methods to probe visual discrimination and its neural basis in head-fixed mice. Mice turn a steering wheel to make two alternative choice, and their behavior matches a simple probabilistic observer. The task engages and requires the visual cortex. Optogenetic stimulation of dopaminergic neurons can replace water control.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
A mesoscale connectome of the mouse brain.
- Record: found
- Abstract: found
- Article: not found
Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance.
- Record: found
- Abstract: not found
- Article: not found