- Record: found
- Abstract: found
- Article: not found
Regulation of Calreticulin Gene Expression by Calcium
Read this article at
Abstract
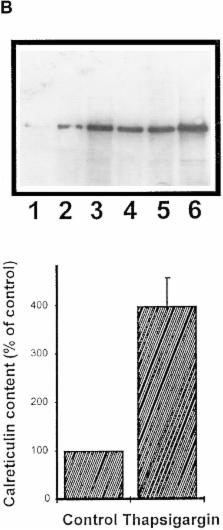
We have isolated and characterized a 12-kb mouse genomic DNA fragment containing the entire calreticulin gene and 2.14 kb of the promoter region. The mouse calreticulin gene consists of nine exons and eight introns, and it spans 4.2 kb of genomic DNA. A 1.8-kb fragment of the calreticulin promoter was subcloned into a reporter gene plasmid containing chloramphenicol acetyltransferase. This construct was then used in transient and stable transfection of NIH/ 3T3 cells. Treatment of transfected cells either with the Ca 2+ ionophore A23187, or with the ER Ca 2+-ATPase inhibitor thapsigargin, resulted in a five- to sevenfold increase of the expression of chloramphenicol acetyltransferase protein. Transactivation of the calreticulin promoter was also increased by fourfold in NIH/3T3 cells treated with bradykinin, a hormone that induces Ca 2+ release from the intracellular Ca 2+ stores. Analysis of the promoter deletion constructs revealed that A23187- and thapsigargin-responsive regions are confined to two regions (−115 to −260 and −685 to −1,763) in the calreticulin promoter that contain the CCAAT nucleotide sequences. Northern blot analysis of cells treated with A23187, or with thapsigargin, revealed a fivefold increase in calreticulin mRNA levels. Thapsigargin also induced a fourfold increase in calreticulun protein levels. Importantly, we show by nuclear run-on transcription analysis that calreticulin gene transcription is increased in NIH/3T3 cells treated with A23187 and thapsigargin in vivo. This increase in gene expression required over 4 h of continuous incubation with the drugs and was also sensitive to treatment with cycloheximide, suggesting that it is dependent on protein synthesis. Changes in the concentration of extracellular and cytoplasmic Ca 2+ did not affect the increased expression of the calreticulin gene. These studies suggest that stress response to the depletion of intracellular Ca 2+ stores induces expression of the calreticulin gene in vitro and in vivo.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
Protein folding in the cell.
- Record: found
- Abstract: found
- Article: not found
Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase.
- Record: found
- Abstract: found
- Article: not found
