- Record: found
- Abstract: found
- Article: found
Iron-induced cytotoxicity mediated by endolysosomal TRPML1 channels is reverted by TFEB
Read this article at
Abstract
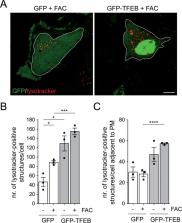
Increased brain iron content has been consistently reported in sporadic Parkinson’s disease (PD) patients, and an increase in cytosolic free iron is known to cause oxidative stress and cell death. However, whether iron also accumulates in susceptible brain areas in humans or in mouse models of familial PD remains unknown. In addition, whilst the lysosome functions as a critical intracellular iron storage organelle, little is known about the mechanisms underlying lysosomal iron release and how this process is influenced by lysosome biogenesis and/or lysosomal exocytosis. Here, we report an increase in brain iron content also in PD patients due to the common G2019S-LRRK2 mutation as compared to healthy age-matched controls, whilst differences in iron content are not observed in G2019S-LRRK2 knockin as compared to control mice. Chemically triggering iron overload in cultured cells causes cytotoxicity via the endolysosomal release of iron which is mediated by TRPML1. TFEB expression reverts the iron overload-associated cytotoxicity by causing lysosomal exocytosis, which is dependent on a TRPML1-mediated increase in cytosolic calcium levels. Therefore, approaches aimed at increasing TFEB levels, or pharmacological TRPML1 activation in conjunction with iron chelation may prove beneficial against cell death associated with iron overload conditions such as those associated with PD.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
A gene network regulating lysosomal biogenesis and function.
- Record: found
- Abstract: found
- Article: not found
The role of iron in brain ageing and neurodegenerative disorders.
- Record: found
- Abstract: found
- Article: not found