- Record: found
- Abstract: found
- Article: found
The use of zebrafish ( Danio rerio) as biomedical models
research-article
Tsegay Teame
1 ,
Zhen Zhang
1 ,
Chao Ran
2 ,
Hongling Zhang
1 ,
Yalin Yang
2 ,
Qianwen Ding
1 ,
Minxu Xie
1 ,
Chenchen Gao
1 ,
Yongan Ye
3 ,
Ming Duan
4 ,
Zhigang Zhou
1
25 June 2019
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Implications
Because of its fully sequenced genome, easy genetic manipulation, high fecundity,
external fertilization and rapid development, and nearly transparent embryo, zebrafish
are a unique model animal for biomedical research, including studies of biological
processes and human diseases.
Zebrafish have all the main organs involved in the process of metabolism and can be
used to study several human metabolic disorders such as nonalcoholic fatty liver disease,
type 2 diabetes mellitus, dyslipidemia, and other hepatic diseases.
With innovation and improvement of molecular techniques, zebrafish will continue to
be an important biomedical model in the future.
Introduction
Various animal species have important roles as experimental models to advance biomedical
research. Animal models provide consistency and validity of research results from
in vitro studies or studies with rodents. Zebrafish has become a popular animal model
for biomedical research. As shown in Figure 1, the number of publications per year
on zebrafish as a model for biomedical research has been significantly increasing
in recent years. One reason that zebrafish are an important biomedical model is because
zebrafish embryos are transparent and they develop outside of the uterus. This unique
developmental process allows scientists to study the details of development starting
from fertilization and continuing throughout development. Innovation and development
of molecular techniques in the later 20th century allowed zebrafish to be used as
a model organism in almost all aspects of biology throughout the world. This review
focuses on the use of zebrafish as a biomedical model in areas mainly related to diet-induced
diseases, metabolic disorders, liver diseases, and intestinal diseases in humans.
Figure 1.
The number of publications in PubMed per year when searching with the keywords “zebrafish”
and “Biomedical.”
Common Fish Species Used as Model Species
For more than 200 years, scientists used fish as model species with goldfish (Carassius
auratus) the oldest model species. Goldfish were primarily used for applied studies
of aquatic toxicology. Additional fish species have also been used, including zebrafish
(Danio rerio), goldfish (Carassius auratus), medaka (Oryzias latipes), roach (Rutilus
rutilus), three-spined stickleback (Gasterosteus aculeatus), pufferfish (Takifugu
rubripes), and the swordtail (Xiphophorus hellerii) (Ribas and Piferrer, 2014). Every
fish species has its unique advantages and disadvantages. For instance, goldfish have
been used to study growth, stress, immunology, and reproduction. Medaka fish were
the most popular species of fish used to study genetics, reproduction, and development.
In recent years, the popularity of zebrafish as a model has increased due to its suitable
features for many research areas.
General Features of Zebrafish
Danio rerio the Latin name for zebrafish formerly called Brachydanio rerio is a small
tropical freshwater fish originating in the Ganges River and its tributaries in northern
India (Tavares and Santos Lopes, 2013). In the natural habitat, zebrafish are usually
found near the bottom of the water to minimize attack by predators. The morphology
of male and female zebrafish is shown in Figure 2.
Figure 2.
Adult male and female AB strain of zebrafish, adapted from https://www.asianscientist.com/2014/12/in-the-lab/zebrafish-switch-sex/
with minor modification.
Currently, zebrafish are considered as a suitable model to investigate development,
genetics, immunity, behavior, physiology, and nutrition. According to its feeding
habits, zebrafish are classified as omnivores and they eat a variety of foods (euryphagous).
During experimental trials, scientists use different types and levels of dietary feeds.
The same amounts of ingredients are used for adult and larvae zebrafish. Moreover,
the feeds and feeding regimes implemented by some laboratories for rearing zebrafish
are varied and, in some cases, are implemented without formal evaluation (Castranova
et al., 2011; Gonzales and Law, 2013).
In the laboratory, to get reasonable research results, zebrafish should receive the
appropriate type and level of dietary nutrients. Most of the time researchers use
different commercial diets for zebrafish, but several commercial diets have undefined
nutritional composition and may have an effect on experimental results (Gonzales and
Law, 2013). In addition, the dietary requirement for larvae and adults are different
in the amount and composition of ingredients. In research studies, it is important
to use a standard diet with adequate nutritional composition and known ingredients,
which promote optimum growth and physiological status of the fish and to minimize
the contribution of unintended nutritional effects on experimental results. The following
diet formulas (Tables 1 and 2) were developed in our laboratory and give consistent
experimental results with zebrafish. We recommend that researchers use these dietary
formulas in their studies with zebrafish.
Table 1.
Dietary formula for zebrafish larvae (5 to 29 d post fertilization)
Basic feed
High sugar
High fat
Low nitrogen
Raw material (g/100 g diet)
Casein
46.00
46.00
46.00
32.00
Gelatin
11.00
11.00
11.00
8.00
Dextrin
22.00
31.00
10.00
32.00
Lard oil
–
–
8.00
–
Soybean oil
3.50
8.00
6.00
Cod liver oil
3.50
2.00
4.00
4.00
Soy lecithin
2.00
2.00
2.00
2.00
Lysine
0.37
0.37
0.37
–
VC phosphate
0.10
0.10
0.10
0.10
Vitamin premix1
0.20
0.20
0.20
0.20
Mineral premix2
0.20
0.20
0.20
0.20
Calcium dihydrogen phosphate
2.00
2.00
2.00
2.00
Choline chloride
0.20
0.20
0.20
0.20
Sodium alginate
4.00
4.00
4.00
4.00
Zeolite powder
4.93
0.93
3.93
9.30
Total
100.00
100.00
100.00
100.00
Proximate composition analysis
Crude protein (estimated)
48.09
48.09
48.09
33.75
Crude fat (estimated)
9.01
4.01
22.01
12.01
Nitrogen-free extract (estimated)
22.00
31.00
10.00
32.00
Total energy (KJ/g)
15.13
14.75
18.02
15.53
1Vitamin premix (g/kg): thiamine, 0.438; riboflavin, 0.632; pyridoxine·HCl, 0.908;
d-pantothenic acid, 1.724; nicotinic acid, 4.583; biotin, 0.211; folic acid, 0.549;
vitamin B12, 0.001; inositol, 21.053; menadione sodium bisulfite, 0.889; retinyl acetate,
0.677; cholecalciferol, 0.116; dl-α-tocopherol-acetate, 12.632.
2Mineral premix (g/kg): CoCl2·6H2O, 0.074; CuSO4·5H2O, 2.5; FeSO4·7H2O, 73.2; NaCl,
40.0; MgSO4·7H2O, 284.0; MnSO4·H2O, 6.50; KI, 0.68; Na2SeO3, 0.10; ZnSO4·7H2O, 131.93;
cellulose, 501.09. (Unpublished data; formulated in our zebrafish laboratory.)
Table 2.
Dietary formula for zebrafish (1 to 3 mo of age)
Basic feed
High Sugar
High fat
Low nitrogen
Raw material (g/100g diet)
Casein
40.00
40.00
40.00
28.00
Gelatin
10.00
10.00
10.00
7.00
Dextrin
28.00
38.00
16.00
38.50
Lard oil
–
–
8.00
–
Soybean oil
6.00
2.00
8.00
6.00
Lysine
0.33
0.33
0.33
–
VC phosphate
0.10
0.10
0.10
0.10
Vitamin premix1
0.20
0.20
0.20
0.20
Mineral premix2
0.20
0.20
0.20
0.20
Calcium dihydrogen phosphate
2.00
2.00
2.00
2.00
Choline chloride
0.20
0.20
0.20
0.20
Sodium alginate
2.00
2.00
2.00
2.00
Microcrystalline cellulose
4.00
4.00
4.00
4.00
Zeolite powder
6.97
0.97
8.97
11.80
Total
100.00
100.00
100.00
100.00
Proximate composition analysis
Crude protein (estimated)
42.19
42.19
42.19
29.53
Crude fat (estimated)
6.01
2.01
16.01
6.01
Nitrogen-free extract (estimated)
28.00
38.00
16.00
38.50
Total energy (KJ/g)
14.02
14.18
15.77
13.65
1Vitamin premix (g/kg): thiamine, 0.438; riboflavin, 0.632; pyridoxine·HCl, 0.908;
d-pantothenic acid, 1.724; nicotinic acid, 4.583; biotin, 0.211; folic acid, 0.549;
vitamin B12, 0.001; inositol, 21.053; menadione sodium bisulfite, 0.889; retinyl acetate,
0.677; cholecalciferol, 0.116; dl-α-tocopherol-acetate, 12.632.
2Mineral premix (g/kg): CoCl2·6H2O, 0.074; CuSO4·5H2O, 2.5; FeSO4·7H2O, 73.2; NaCl,
40.0; MgSO4·7H2O, 284.0; MnSO4·H2O, 6.50; KI, 0.68; Na2SeO3, 0.10; ZnSO4·7H2O, 131.93;
cellulose, 501.09. (Unpublished data; formulated in our zebrafish laboratory.)
The amount of feed varies across the different growth stages of the fish and is dependent
on the stage of growth. From 5 d post fertilization, zebrafish larvae are mostly fed
zooplanktons such as paramecium and rotifers and young larvae can be fed with artificial
food up to 100 μm in size or live feed. For adult fish, the size of the dry food can
range from 300 to 400 μm (Avdesh et al., 2012). The size of the dry food can increase
with increasing size of the fish. The commonly practiced feeding ratio of zebrafish
is about 4% of its bodyweight. Overfeeding may increase the concentration of nitrate
in the water and affect the physiology of the fish. In addition, overeating may cause
death of the fish.
Why Do Zebrafish Make Such Good Animal Models?
The criteria to select animal models for biomedical research are directly related
to the final goal of the research. The use of zebrafish as a biomedical model was
suggested by George Streisinger and colleagues at the University of Oregon, who launched
the modern era for zebrafish in the field of biomedical research (Clark and Ekker,
2015). Zebrafish are popular animal models because they have numerous advantages over
other species. The most advantageous features of zebrafish are a fully sequenced genome,
easy manipulation of its genome, high fecundity, short generation time (about 3 mo),
rapid embryonic development (24 hr), and external fertilization. The translucent zebrafish
embryo allows study of the different developmental stages starting from the early
stage of embryogenesis. In addition, zebrafish embryos form complete organ systems,
including heart, intestine and blood vessels within 48 hr after fertilization. More
than 10,000 mutants in protein-coding genes have been generated (Howe et al., 2013)
and several transgenic lines of zebrafish have been made to study human diseases.
The availability of multiple strains of zebrafish is another important advantage of
this species. In addition, it is also very affordable to maintain a large number of
zebrafish in a relatively small amount of laboratory space. Although zebrafish require
relatively easy management, special attention must be paid to ensuring a healthy diet
and adequate water quality to optimize fish health and growth. While there are several
strains of zebrafish in the world, the most widely used strains in biomedical research
are AB, Casper, Ekkwill, Nadia, Wild Indian Karyotype, wild-caught, and Tubingen.
According to the ZFIN website, more than 800 biological laboratories around the world
conduct basic and applied research with zebrafish (https://zfin.org/search?q=Zebrafish+laboratories&category).
Many of these laboratories use zebrafish to study human diseases, including neural
disorders, cancer, infectious diseases, cardiovascular diseases, kidney diseases,
diabetes, blindness, deafness, digestive diseases, hematopoiesis, and muscle disorders.
Mutant zebrafish have been established by knocking out or knocking in specific genes.
These alterations create novel biomedical models. For example, if the patient has
a disease related to metabolism, different mutations in zebrafish genes related to
metabolism can be made and then changes in gene expression can be monitored using
different molecular techniques. The short generation time of zebrafish makes it difficult
to produce stable transgenic adults or homozygous mutant embryos, which usually requires
about 4 months. Recently, scientists have developed many technologies to expedite
the transgenic process (Burger et al., 2016). The presence or absence of genomic duplication
events in zebrafish makes it complicated to study some human diseases such as diabetes
mellitus. Zebrafish are also important for developing new therapies or screening novel
drugs to treat or prevent human diseases.
Even though zebrafish are an important biomedical model, they have some limitations,
including the dissimilarity of some organs like the respiratory system and the reproductive
system. Thus, it is difficult to use zebrafish as a model for respiration or reproduction
in humans. In addition, because zebrafish live in an aquatic habitat, screening of
some water soluble drugs in zebrafish is another limitation.
Zebrafish as a Model for Metabolic Diseases
There are several examples of human diseases that have been successfully modeled in
zebrafish such as Duchenne muscular dystrophy, human melanoma, acute lymphoblastic
leukemia, polycystic kidney disease, nephronophthisis, acute kidney injury, Parkinson’s
disease, Huntington’s disease, Alzheimer disease, myocardial infarction, and some
metabolic diseases. As shown in Figure 3, in addition to genomic similarity, the presence
of conserved organs and organ systems between human and zebrafish contributes to development
of a number of successful models of human diseases.
Figure 3.
Some of the conserved organ systems between zebrafish and humans (adapted from http://www.intl.upm.edu.my/article/zebrafish_replace_lab_rat-30977
with minor modification).
We will focus on the common human metabolic diseases successfully modeled in zebrafish,
including obesity, type 2 diabetes mellitus, nonalcoholic steatohepatitis, and atherosclerosis.
Disturbance of the normal process of converting food to energy in the cell results
in different metabolic disorders. Even though zebrafish and humans have differences
in basic nutrient requirements, different metabolic mechanisms may not be needed.
To keep the balance between the production and utilization of energy several organs
are involved, including the brain, intestines, liver, skeletal muscle, and adipose
tissue. Whole animal models are needed to study the entire process of metabolism.
Zebrafish are an appropriate model to study metabolic dysfunction because they have
all the organs involved in energy homeostasis and metabolism including appetite and
insulin regulation and a lipid storage system which is conserved with that found in
humans (Nishio et al., 2012).
A report from World Health Organization indicated that, of the metabolism-related
human diseases, cardiovascular disease is currently the most predominant fatal disease
(Lozano et al., 2012). Obesity (Ng et al., 2014), type 2 diabetes mellitus, and nonalcoholic
fatty liver disease (LaBrecque et al., 2014) increase the risk of cardiovascular disease.
Because zebrafish and humans have similar metabolic organs (including the digestive
organs, adipose tissue, and muscle), zebrafish are a popular model to study metabolic
disorders. In addition, the availability of several new tools and approaches such
as talens, CRISPR/Cas9 (Wu et al., 2018), compound treatment (Poureetezadi et al.,
2016), mass spectrometry-based polar metabolomics and lipidomics (Zhang et al., 2018),
and in vivo imaging of fluorescent dyes (Minchin et al., 2018) make it possible to
investigate the molecular mechanisms of metabolic processes in zebrafish.
Researchers have also used zebrafish as a model organism to study different types
of metabolic diseases such as congenital errors of metabolism, hyper- and hypothyroidism,
disorders of the hypothalamus–pituitary–adrenal axis, dysregulation of the circadian
clock, and cancer metabolism (Gut et al., 2017). In this review, our emphasis will
be on diet-induced metabolic disorders.
Zebrafish as a Model Animal for Diet-induced Obesity
Utilization of zebrafish in diet-induced obesity studies was first developed by Oka
et al. (2010) by feeding adult zebrafish Artemia nauplii. In these studies, the fish
showed increased body mass index, developed hepatic steatosis, hypertriglyceridemia,
and dysregulation of some lipid metabolism genes. Chen et al. (2018) fed zebrafish
a diet of high cholesterol, which resulted in increased body weight, increased triglyceride
levels, and lipid deposition in the liver. Over nutrition of zebrafish with high fat
from different sources or cholesterol also lead to hyperglycemia and ectopic lipid
accumulation, increased body weight, increased adipose tissue, cardiovascular overload,
and steatosis (Forn-Cuní et al., 2015). Landgraf et al. (2017) used zebrafish to compare
the result of overfeeding with normal and high-fat diets on obesity development. They
concluded that both diets showed an increase in adipose tissue and the fish fed the
normal fat diet developed obesity, but these fish were metabolically healthy. The
other fish fed a high-fat diet were unhealthy. Similar with the above findings, in
our laboratory, we also found that larvae and adult zebrafish fed a high-fat diet
developed hepatic steatosis as shown in Figure 4. In zebrafish, diet-induced obesity
is also used to estimate the type of food and effect of nutrient compounds on development,
testing, and discovering different drugs to prevent or treat obesity and by altering
fat metabolism. The diet-induced obesity zebrafish model overfed with Artemia shares
common pathophysiological pathways with mammalian obesity and can be used to identify
putative pharmacological targets of human obesity (Oka et al., 2010). Therefore, the
diet-induced obesity approach allows us to understand the disease in the context of
systematic obesity, hence mimicking the most common process occurring in humans affected
by this condition.
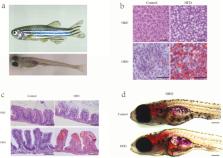
Figure 4.
High-fat diets (HFD) induced hepatic steatosis in adult and larval zebrafish. (a)
Adult zebrafish (1 mo old) and larval zebrafish (5 d post fertilization). (b) Representative
liver histology image by Haemotoxylin and Eosin (H&E) staining and oil red O (ORO)
staining of adult zebrafish fed with a control diet or HFD for 4 wk. The scale bar
is 50 μm. (c) Representative intestinal histology image by H&E staining and ORO staining
of adult zebrafish fed with a control diet or HFD for 4 wk. The scale bar is 100 μm.
(d) Representative image of whole-mount ORO staining in zebrafish larvae fed control
diet and HFD for 7 d. The scale bar is 200 μm. (Unpublished data from our zebrafish
laboratory.)
Zebrafish as Model for Glucose Metabolism and Type 2 Diabetes Mellitus
The main cause for development of diabetes mellitus is the failure of pancreatic β-cells
to produce insulin, which leads to insulin deficiency. These functions and processes
are conserved between zebrafish and humans. Zebrafish exposure to hypercaloric and
high-fat diets quickly induces obesity and obesity‐related disease, and activates
metabolic pathways very similar to their human counterparts. If glucose is available
in the diet, insulin is produced by the pancreas, and gluconeogenesis is inhibited
through the down-regulation of genes involved in the pathway. In the absence of glucose
in the bloodstream, gluconeogenesis is induced by the action of glucagon. Capiotti
et al. (2014) revealed that zebrafish immersed in a high-glucose solution (111 mM)
for 14 d were able to increase by 41% froctosamine (glycated protein) levels from
the eyes, decreased amounts of mRNA for insulin receptors in muscle, and developed
hyperglycemia. Zang et al. (2017) developed a zebrafish model of type 2 diabetes mellitus
by overfeeding a high-calorie diet (408 calories per fish per day). Using gene expression
profiling in the liver and pancreas, a common pathway for development of type 2 diabetes
mellitus was seen between zebrafish and humans. The relationship between age and type
2 diabetes mellitus was developed by Connaughton et al. (2016) and revealed that young
zebrafish (4 to 11 mo olds) developed hyperglycemia slower than old zebrafish with
increasing concentrations of glucose. The glucose concentration of homeostasis organs
can be increased by immersing zebrafish embryos in a glucose solution. Gleeson et
al. (2007) showed that immersion of adult zebrafish in a 1% glucose solution for 24
hr increase blood glucose up to 400 mg/dL. The two transgenic models of insulin resistance
established by Zang et al. (2017) were skeletal muscle insulin resistance achieved
by transgenic expression of a dominant-negative IGF-I receptor in skeletal muscle.
In the second model, insulin resistance was attained via liver--specific knockdown
of the insulin receptor gene using CRISPR/Cas9 (Yin et al., 2015). These results revealed
that zebrafish are a suitable model to study glucose-induced human disease. Marín-Juez
et al. (2014) also developed a zebrafish model for hyperinsulinemia by injecting human
recombinant insulin in zebrafish larvae. These studies demonstrated upregulation of
the negative immune modulator protein tyrosine phosphatase non receptor type 6 in
insulin-resistant larvae. Recent research results of Yang et al. (2018) showed that
mutant zebrafish with a knockout in insulin receptor a and b genes when fed a high-carbohydrate
(41%) diet showed hyperglycemia, reduced growth hormone signaling, increased visceral
adiposity, and fatty liver development, which are similar signs to the human lipodystrophy
disease. The glucose level in zebrafish can be measured using two hand-held glucose
meters designed for use in humans with diabetics (Eames et al., 2010). Additionally,
fasting for performing postprandial glucose and intraperitoneal glucose tolerance
tests can be used. There are several methods of measuring insulin levels in zebrafish,
including measuring the insulin mRNA expression level by q-PCR (Michel et al., 2016),
insulin antibody for immunostaining (Kimmel et al., 2015), or semi-quantitative dot-blot
(Olsen et al., 2012). Insulin sensitivity can also be assessed by intraperitoneal
injection of insulin in hyperglycemic zebrafish (Capiotti et al., 2014).
Zebrafish as Model for Dyslipidemia and Atherosclerosis Diseases
Increasing the level of cholesterol, triglycerides, or high-density lipoprotein cholesterol
resulted in dyslipidemia, and in turn, led to development of atherosclerosis. Since
the nutritional requirements of zebrafish are known, several researchers established
different models by changing the standard diet (such as feeding zebrafish a high-fat
diet to develop obesity, hyperglycemia, and dyslipidemia) to induce metabolic stress
on the fish. The histopathological changes showed by zebrafish fed a high level of
cholesterol are very similar with the symptoms shown in human atherosclerosis (Fang
and Miller, 2012). Formulation of a high-cholesterol diet is also important for the
study of dyslipidemia (Oka et al., 2010). Miyares et al. (2014) described lipid and
lipoprotein metabolism using the zebrafish embryo yolk metabolism stages and concluded
that incorporation of exogenous fatty acids into the circulatory system was dependent
on lipoprotein production in the system.
Zebrafish as a Model for Nonalcoholic Fatty Liver Disease and Other Liver Disorders
Nonalcoholic fatty liver disease is not related to overconsumption of alcohol. It
is the accumulation of excess fat in the liver, and this can lead to steatosis, steatohepatitis,
fibrosis, corrihosis, and hepatocellular carcinoma. This disease can develop and be
associated with insulin resistance, high-fat diets, drug-induced liver injuries, and
metabolic syndromes. Several research results show that zebrafish also develop hepatic
steatosis when exposed to hepatotoxic chemicals, fasting and excessive dietary fat,
cholesterol, or carbohydrate. These mechanisms are similar in zebrafish and humans.
Interestingly, publication of the first paper on zebrafish development (Roosen, 1937)
investigated the effect of different toxins, alcohol, and different levels of carbohydrate
or fat diets on zebrafish embryos, larvae, and adult developmental stages. The application
of toxins to the fish tank is a simple technique and this technique makes zebrafish
a popular model to study chemical screening mechanisms.
The zebrafish liver resembles the human liver in cellular structure, function, and
genetics. This observation led investigators to use zebrafish to study the detailed
embryological and genetics associated with development of the human liver, as well
as liver disorders and potential therapies for liver diseases. Development of liver
tumors in zebrafish using carcinogenic substances and comparison with gene expression
in tumors of human livers first pointed to the importance of zebrafish as an appropriate
biomedical model. Tonin et al. (2018) showed that zebrafish immersed in 6% fructose
lead to the formation of hepatic steatosis in a manner similar to the symptoms shown
in humans fed a high-carbohydrate diet. Using a differential feeding strategy, Yang
et al. (2019) showed that over feeding resulted in development of fatty liver and
hastened the carcinogenic process. In addition, the hormone leptin, which is responsible
for obesity, was unregulated in the oncogenic and overfed zebrafish. They also found
that, by downregulating leptin signaling, it is possible to reduce the muscle wasting
phenotype. Development of a mutated gene foie gras in zebrafish initiated scientists
to study development of hepatic steatosis and the associated molecular mechanisms.
In addition, development of gonzo mutant zebrafish showed that development of alcohol-induced
hepatic steatosis was mediated by sterol response element binding protein transcription
factors (Passeri et al., 2009). Shimada et al. (2015) applied transcriptomic and proteomic
methods using a model of diet-induced obesity in the liver of zebrafish to isolate
genes responsible for the formation of hepatic steatosis. In these studies, fatty
acid binding protein 3 and transcription factors (E2F) were upregulated in hepatic
steatosis zebrafish. Howarth et al. (2013) developed two models using zebrafish to
investigate either tunicamycin- or ethanol-provoked steatosis which leads to liver
failure. They prevented ethanol-induced steatosis by blocking activation of sterol
response element binding proteins using mutant zebrafish. In these studies, even without
lipid accumulation, hepatocyte dysfunction occurred. Recent research from Imran et
al. (2018) using zebrafish larvae to test the involvement of membrane remodeling in
hepatotoxicity showed that co-exposure of obese zebrafish larvae to benzo[a]pyrene
and ethanol induced in vivo hepatotoxicity through membrane remodeling. This result
led scientists to develop a therapy for nonalcoholic fatty liver disease and associated
risk factors.
Zebrafish as a Model for the Study of Intestinal Diseases and Host–Microbe Interactions
The intestine of zebrafish is a long tube like structure, which has been divided into
the intestine bulb, mid-intestine, and posterior intestine that folds twice in the
abdominal cavity. The absorptive enterocytes, goblet cells, and endocrine cells are
the three cell types that have differentiated from the intestine epithelium. Since
innovation of forward genetic screening techniques, many scientists have used zebrafish
as a model to study the physiology, function, and diseases of the human intestine.
The entire intestinal track opens at 6 d post fertilization and at this time larvae
start to feed on small aquatic animals (Brugman, 2016). At this stage of development,
the intestine of the fish is easily visible and its morphology can be observed with
a microscope. Because of its transparent body, many researchers have developed a zebrafish
model of intestinal inflammation. Ji et al. (2018) developed a zebrafish model to
evaluate how bioactive compounds are taken up by the intestine. They concluded that
bioactive compounds are able to cross the intestinal mucosal barriers and pass through
the lamina propria to reach the muscle. Arias-Jayo et al. (2018) showed that zebrafish
fed a high-fat diet of 10% (w/w) cocoa butter added to the normal diet resulted in
intestinal inflammation via activation of NF-κβ. The intestinal barrier was also damaged
and there was an increase in mucin production by goblet cells. Oehlers et al. (2011a)
developed a model with zebrafish embryos infected with salmonella, and showed that
depletion of the bacterial detector proteins NOD1 and NOD2 reduced expression of the
dual oxidase in the intestinal epithelial. This also weakened the ability of the fish
to reduce the intracellular burden of bacteria. Overall, this finding was a good model
for Crohn’s disease in humans.
Zebrafish have also been used to study host–microbe interactions in the digestive
system. Recent studies of the intestinal microbiome in zebrafish with a mutation in
gene myd88 demonstrated that changes due to the microbiome in the body (especially
the intestinal leukocytes) are dependent on the immune adaptor gene myd88 (Koch et
al., 2018). Raising germ-free zebrafish to investigate the effect of microbiota on
the innate immune system has also been studied (Kostic et al., 2013) and the contribution
of gut microbes on fatty acid absorption was studied by Semova et al. (2012). In these
studies with zebrafish, the fish with microbes in their gut had increased fatty acid
absorption, higher accumulation of fats in the liver and the body when compared with
germ-free zebrafish. The gut microbiota of human and zebrafish are different. Valenzuela
et al. (2018) showed that germ-free zebrafish larvae can be colonized by human gut
microorganisms, such as Clostridioides difficile and Bacillus. This result opened
an interesting area to study interactions between these microorganisms and the host.
The role of the gut microbiota on host biology is similar between zebrafish and mammals
and, in both species, intestinal microbiota participate in the education of the immune
system, maturation of the gut, and promotion of nutrient metabolism in the host (Bates
et al., 2007).Therefore, zebrafish are an important model to further explore intestinal
diseases and related aspects of gut biology.
Conclusion
Zebrafish are an important biomedical model in every aspect of biology. Zebrafish
have several suitable features for developmental, physiological, and genetic studies
including external fertilization and the transparent nature of embryo. The large degree
of functional conservation of morphology, genetics, and physiology between zebrafish
and humans makes zebrafish an attractive model for several human disorders and development
of potential therapies for humans. Advancement of nanotechnologies and molecular techniques
also contributes to the use of zebrafish to study different diseases in humans. In
this review, we emphasized some biomedical areas where zebrafish are a popular model
to investigate the mechanisms and processes associated with metabolic diseases, including
diet-induced obesity, type 2 diabetes mellitus, dyslipidemia and atherosclerosis,
liver-related diseases, and intestinal diseases. Scientists have also used zebrafish
to develop new therapies to treat and prevent these important human diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31872584,
3180131599, 31702354, 31602169, 31672294, 31572633), the Beijing earmarked fund for
Modern Agro-industry Technology Research System (SCGWZJ 20191104-4), and Innovation
Capability Support Program of Shaanxi (2018TD-021).
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013.
Marie Ng, Tom Fleming, Margaret W. Robinson … (2015)
- Record: found
- Abstract: found
- Article: not found
The zebrafish reference genome sequence and its relationship to the human genome.
Kerstin Howe, Matthew D. Clark, Carlos F Torroja … (2013)
- Record: found
- Abstract: found
- Article: not found
Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota.
Jennifer Bates, Janie Akerlund, Erika Mittge … (2007)