- Record: found
- Abstract: found
- Article: found
Non-invasive Imaging and Modeling of Liver Regeneration After Partial Hepatectomy
Read this article at
Abstract
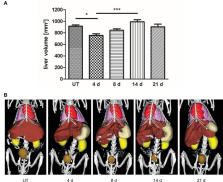
The liver has a unique regenerative capability upon injury or partial resection. The regeneration process comprises a complex interplay between parenchymal and non-parenchymal cells and is tightly regulated at different scales. Thus, we investigated liver regeneration using multi-scale methods by combining non-invasive imaging with immunohistochemical analyses. In this context, non-invasive imaging can provide quantitative data of processes involved in liver regeneration at organ and body scale. We quantitatively measured liver volume recovery after 70% partial hepatectomy (PHx) by micro computed tomography (μCT) and investigated changes in the density of CD68 + macrophages by fluorescence-mediated tomography (FMT) combined with μCT using a newly developed near-infrared fluorescent probe. In addition, angiogenesis and tissue-resident macrophages were analyzed by immunohistochemistry. Based on the results, a model describing liver regeneration and the interactions between different cell types was established. In vivo analysis of liver volume regeneration over 21 days after PHx by μCT imaging demonstrated that the liver volume rapidly increased after PHx reaching a maximum at day 14 and normalizing until day 21. An increase in CD68 + macrophage density in the liver was detected from day 4 to day 8 by combined FMT-μCT imaging, followed by a decline towards control levels between day 14 and day 21. Immunohistochemistry revealed the highest angiogenic activity at day 4 after PHx that continuously declined thereafter, whereas the density of tissue-resident CD169 + macrophages was not altered. The simulated time courses for volume recovery, angiogenesis and macrophage density reflect the experimental data describing liver regeneration after PHx at organ and tissue scale. In this context, our study highlights the importance of non-invasive imaging for acquiring quantitative organ scale data that enable modeling of liver regeneration.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Liver regeneration: from myth to mechanism.
- Record: found
- Abstract: found
- Article: not found
Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas.
- Record: found
- Abstract: found
- Article: not found