- Record: found
- Abstract: found
- Article: found
Clinical development of CAR T cell therapy in China: 2020 update
Read this article at
Abstract
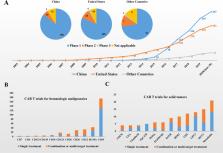
Chimeric antigen receptor (CAR) T-cell therapy has achieved significant success in the treatment of hematological malignancies. In recent years, fast-growing CAR T clinical trials have actively explored their potential application scenarios. According to the data from the clinicaltrials.gov website, China became the country with the most registered CAR T trials in September 2017. As of June 30, 2020, the number of registered CAR T trials in China has reached 357. In addition, as many as 150 other CAR T trials have been registered on ChiCTR. Although CAR T therapy is flourishing in China, there are still some problems that cannot be ignored. In this review, we aim to systematically summarize the clinical practice of CAR T-cell therapy in China. This review will provide an informative reference for colleagues in the field, and a better understanding of the history and current situation will help us more reasonably conduct research and promote cooperation.
Related collections
Most cited references122
- Record: found
- Abstract: found
- Article: not found
Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma
- Record: found
- Abstract: found
- Article: not found
Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma
- Record: found
- Abstract: found
- Article: not found