- Record: found
- Abstract: found
- Article: found
Toxicological Effects of Cadmium on Mammalian Testis
Read this article at
Abstract
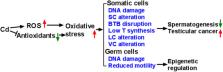
Cadmium is a heavy metal, and people are exposed to it through contaminated foods and smoking. In humans and other mammals, cadmium causes damage to male testis. In this review, we summarize the effects of cadmium on the development and function of the testis. Cadmium causes severe structural damage to the seminiferous tubules, Sertoli cells, and blood-testis barrier, thus leading to the loss of sperm. Cadmium hinders Leydig cell development, inhibits Leydig cell function, and induces Leydig cell tumors. Cadmium also disrupts the vascular system of the testis. Cadmium is a reactive oxygen species inducer and possibly induces DNA damage, thus epigenetically regulating somatic cell and germ cell function, leading to male subfertility/infertility.
Related collections
Most cited references105
- Record: found
- Abstract: found
- Article: not found
Cadmium: toxic effects on the reproductive system and the embryo.

- Record: found
- Abstract: found
- Article: found
The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty
- Record: found
- Abstract: found
- Article: not found