- Record: found
- Abstract: found
- Article: found
Double-edged-sword effect of IL-1 β on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF- κB, MAPK and BMP/Smad signaling pathways
Read this article at
Abstract
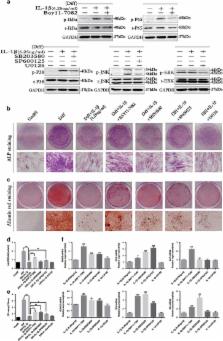
Microenvironmental conditions can interfere with the functional role and differentiation of mesenchymal stem cells (MSCs). Recent studies suggest that an inflammatory microenvironment can significantly impact the osteogenic potential of periodontal ligament stem cells (PDLSCs), but the precise effects and mechanisms involved remain unclear. Here, we show for the first time that interleukin-1 β (IL-1 β) has dual roles in the osteogenesis of PDLSCs at concentrations ranging from physiologically healthy levels to those found in chronic periodontitis. Low doses of IL-1 β activate the BMP/Smad signaling pathway to promote the osteogenesis of PDLSCs, but higher doses of IL-1 β inhibit BMP/Smad signaling through the activation of nuclear factor- κB (NF- κB) and mitogen-activated protein kinase (MAPK) signaling, inhibiting osteogenesis. These results demonstrate that crosstalk between NF- κB, MAPK and BMP/Smad signaling mediates this dual effect of IL-1 β on PDLSCs. We also show that the impaired osteogenesis of PDLSCs results in more inflammatory cytokines and chemokines being released, inducing the chemotaxis of macrophages, which further clarifies the role of PDLSCs in the pathogenesis of periodontitis.
Related collections
Most cited references33

- Record: found
- Abstract: found
- Article: found
TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation
- Record: found
- Abstract: found
- Article: not found
Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine.
- Record: found
- Abstract: found
- Article: not found