- Record: found
- Abstract: found
- Article: found
Targeting nuclear hormone receptors for the prevention of breast cancer
Read this article at
Abstract
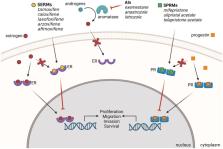
Advancements in research have led to the steady decline of breast cancer mortality over the past thirty years. However, breast cancer incidence has continued to rise, resulting in an undue burden on healthcare costs and highlighting a great need for more effective breast cancer prevention strategies, including targeted chemo preventative agents. Efforts to understand the etiology of breast cancer have uncovered important roles for nuclear receptors in the development and progression of breast cancer. Targeted therapies to inhibit estrogen receptor (ER) and progesterone receptor (PR) signaling (selective ER modulators, aromatase inhibitors and selective PR modulators) have shown great promise for the treatment and prevention of hormone receptor (HR)-positive breast cancer. However, these drugs do not prevent HR-negative disease. Therefore, recent efforts have focused on novel targeted therapies with the potential to prevent both HR-positive and HR-negative breast cancer. Among these include drugs that target other nuclear receptors, such as retinoic acid receptor (RAR), retinoid X receptor (RXR) and vitamin D receptor (VDR). In this review we provide an overview of recent preclinical and clinical trials targeting members of the nuclear receptor superfamily for the prevention of breast cancer.
Related collections
Most cited references197
- Record: found
- Abstract: found
- Article: not found
Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation.
- Record: found
- Abstract: found
- Article: not found