- Record: found
- Abstract: found
- Article: found
Unravelling HP1 functions: post-transcriptional regulation of stem cell fate
Read this article at
Abstract
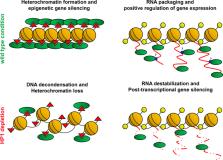
Heterochromatin protein 1 (HP1) is a non-histone chromosomal protein first identified in Drosophila as a major component of constitutive heterochromatin, required for stable epigenetic gene silencing in many species including humans. Over the years, several studies have highlighted additional roles of HP1 in different cellular processes including telomere maintenance, DNA replication and repair, chromosome segregation and, surprisingly, positive regulation of gene expression. In this review, we briefly summarize past research and recent results supporting the unexpected and emerging role of HP1 in activating gene expression. In particular, we discuss the role of HP1 in post-transcriptional regulation of mRNA processing because it has proved decisive in the control of germline stem cells homeostasis in Drosophila and has certainly added a new dimension to our understanding on HP1 targeting and functions in epigenetic regulation of stem cell behaviour.
Related collections
Most cited references110
- Record: found
- Abstract: found
- Article: not found
Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain.
- Record: found
- Abstract: found
- Article: not found
Phase separation drives heterochromatin domain formation
- Record: found
- Abstract: found
- Article: not found