- Record: found
- Abstract: found
- Article: found
Uniform nomenclature for the mitochondrial contact site and cristae organizing system
review-article
Nikolaus Pfanner
1
,
2
,
,
Martin van der Laan
1
,
2 ,
Paolo Amati
3 ,
Roderick A. Capaldi
4 ,
Amy A. Caudy
5
,
6 ,
Agnieszka Chacinska
7 ,
Manjula Darshi
8 ,
Markus Deckers
11 ,
Suzanne Hoppins
12 ,
Tateo Icho
13 ,
Stefan Jakobs
14
,
15 ,
Jianguo Ji
16 ,
Vera Kozjak-Pavlovic
17 ,
Chris Meisinger
1
,
2 ,
Paul R. Odgren
18 ,
Sang Ki Park
19 ,
Peter Rehling
11
,
15 ,
Andreas S. Reichert
20
,
21 ,
M. Saeed Sheikh
22 ,
Susan S. Taylor
8
,
9
,
10 ,
Nobuo Tsuchida
23 ,
Alexander M. van der Bliek
24 ,
Ida J. van der Klei
25 ,
Jonathan S. Weissman
26
,
27 ,
Benedikt Westermann
28 ,
Jiping Zha
29 ,
Walter Neupert
30
,
,
Jodi Nunnari
31
,
31 March 2014
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
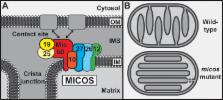
The mitochondrial inner membrane contains a large protein complex that functions in inner membrane organization and formation of membrane contact sites. The complex was variably named the mitochondrial contact site complex, mitochondrial inner membrane organizing system, mitochondrial organizing structure, or Mitofilin/Fcj1 complex. To facilitate future studies, we propose to unify the nomenclature and term the complex “mitochondrial contact site and cristae organizing system” and its subunits Mic10 to Mic60.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Macromolecular organization of ATP synthase and complex I in whole mitochondria.
Adriana Rycovska, Bertram Daum, Volker Zickermann … (2011)
- Record: found
- Abstract: found
- Article: not found
The mitochondrial contact site complex, a determinant of mitochondrial architecture.
Dirk Walther, Ulrich Welsch, Matthias Mann … (2011)