- Record: found
- Abstract: found
- Article: not found
Methionine‐deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF‐I and insulin levels, and increases hepatocyte MIF levels and stress resistance
Read this article at
Summary
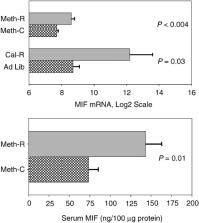
A diet deficient in the amino acid methionine has previously been shown to extend lifespan in several stocks of inbred rats. We report here that a methionine‐deficient (Meth‐R) diet also increases maximal lifespan in (BALB/cJ × C57BL/6 J)F1 mice. Compared with controls, Meth‐R mice have significantly lower levels of serum IGF‐I, insulin, glucose and thyroid hormone. Meth‐R mice also have higher levels of liver mRNA for MIF (macrophage migration inhibition factor), known to be higher in several other mouse models of extended longevity. Meth‐R mice are significantly slower to develop lens turbidity and to show age‐related changes in T‐cell subsets. They are also dramatically more resistant to oxidative liver cell injury induced by injection of toxic doses of acetaminophen. The spectrum of terminal illnesses in the Meth‐R group is similar to that seen in control mice. Studies of the cellular and molecular biology of methionine‐deprived mice may, in parallel to studies of calorie‐restricted mice, provide insights into the way in which nutritional factors modulate longevity and late‐life illnesses.
Related collections
Most cited references16
- Record: found
- Abstract: not found
- Article: not found
Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging.
- Record: found
- Abstract: found
- Article: not found
Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline.
- Record: found
- Abstract: found
- Article: not found
