- Record: found
- Abstract: found
- Article: found
NANOS3 function in human germ cell development
Read this article at
Abstract
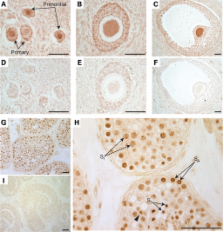
Human infertility is common and frequently linked to poor germ cell development. Yet, human germ cell development is poorly understood, at least in part due to the inaccessibility of germ cells to study especially during fetal development. Here, we explored the function of a highly conserved family of genes, the NANOS genes, in the differentiation of human germ cells from human embryonic stem cells. We observed that NANOS-1, -2 and -3 mRNAs and proteins were expressed in human gonads. We also noted that NANOS3 was expressed in germ cells throughout spermatogenesis and oogenesis and thus, focused further efforts on this family member. NANOS3 expression was highest in human germ cell nuclei where the protein co-localized with chromosomal DNA during mitosis/meiosis. Reduced expression of NANOS3 (via morpholinos or short hairpin RNA) resulted in a reduction in germ cell numbers and decreased expression of germ cell-intrinsic genes required for the maintenance of pluripotency and meiotic initiation and progression. These data provide the first direct experimental evidence that NANOS3 functions in human germ cell development; indeed, NANOS3 is now one of just two genes that has been directly shown to function in germ cell development across diverse species from flies, worms, frogs and mice to humans [the other is BOULE, a member of the Deleted in Azoospermia (DAZ) gene family]. Findings may contribute to our understanding of the basic biology of human germ cell development and may provide clinical insights regarding infertility.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
The rapid evolution of reproductive proteins.
- Record: found
- Abstract: found
- Article: not found
Bmp4 is required for the generation of primordial germ cells in the mouse embryo.
- Record: found
- Abstract: found
- Article: not found