- Record: found
- Abstract: found
- Article: found
Gene Conversion-Like Events in the Diversification of Human Rearranged IGHV3-23*01 Gene Sequences
Read this article at
Abstract
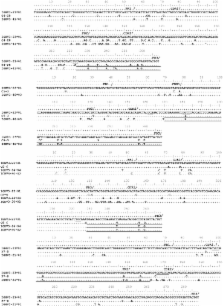
Gene conversion (GCV), a mechanism mediated by activation-induced cytidine deaminase (AID) is well established as a mechanism of immunoglobulin diversification in a few species. However, definitive evidence of GCV-like events in human immunoglobulin genes is scarce. The lack of evidence of GCV in human rearranged immunoglobulin gene sequences is puzzling given the presence of highly similar germline donors and the presence of all the enzymatic machinery required for GCV. In this study, we undertook a computational analysis of rearranged IGHV3-23 *01 gene sequences from common variable immunodeficiency (CVID) patients, AID-deficient patients, and healthy individuals to survey “GCV-like” activities. We analyzed rearranged IGHV3-23 *01 gene sequences obtained from total PBMC RNA and single-cell polymerase chain reaction of individual B cell lysates. Our search identified strong evidence of GCV-like activity. We observed that GCV-like tracts are flanked by AID hotspot motifs. Structural modeling of IGHV3-23 *01 gene sequence revealed that hypermutable bases flanking GCV-like tracts are in the single stranded DNA (ssDNA) of stable stem-loop structures (SLSs). ssDNA is inherently fragile and also an optimal target for AID. We speculate that GCV could have been initiated by the targeting of hypermutable bases in ssDNA state in stable SLSs, plausibly by AID. We have observed that the frequency of GCV-like events is significantly higher in rearranged IGHV3-23- *01 sequences from healthy individuals compared to that of CVID patients. We did not observe GCV-like events in rearranged IGHV3-23- *01 sequences from AID-deficient patients. GCV, unlike somatic hypermutation (SHM), can result in multiple base substitutions that can alter many amino acids. The extensive changes in antibody affinity by GCV-like events would be instrumental in protecting humans against pathogens that diversify their genome by antigenic shift.
Related collections
Most cited references114
- Record: found
- Abstract: found
- Article: not found
Somatic generation of antibody diversity.
- Record: found
- Abstract: found
- Article: not found
Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme.
- Record: found
- Abstract: found
- Article: not found