- Record: found
- Abstract: found
- Article: found
Deciphering the intracellular metabolism of Listeria monocytogenes by mutant screening and modelling
Read this article at
Abstract
Background
The human pathogen Listeria monocytogenes resides and proliferates within the cytoplasm of epithelial cells. While the virulence factors essentially contributing to this step of the infection cycle are well characterized, the set of listerial genes contributing to intracellular replication remains to be defined on a genome-wide level.
Results
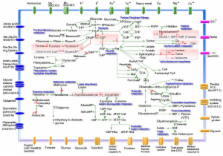
A comprehensive library of L. monocytogenes strain EGD knockout mutants was constructed upon insertion-duplication mutagenesis, and 1491 mutants were tested for their phenotypes in rich medium and in a Caco-2 cell culture assay. Following sequencing of the plasmid insertion site, 141 different genes required for invasion of and replication in Caco-2 cells were identified. Ten in-frame deletion mutants were constructed that confirmed the data. The genes with known functions are mainly involved in cellular processes including transport, in the intermediary metabolism of sugars, nucleotides and lipids, and in information pathways such as regulatory functions. No function could be ascribed to 18 genes, and a counterpart of eight genes is missing in the apathogenic species L. innocua. Mice infection studies revealed the in vivo requirement of IspE (Lmo0190) involved in mevalonate synthesis, and of the novel ABC transporter Lmo0135-0137 associated with cysteine transport. Based on the data of this genome-scale screening, an extreme pathway and elementary mode analysis was applied that demonstrates the critical role of glycerol and purine metabolism, of fucose utilization, and of the synthesis of glutathione, aspartate semialdehyde, serine and branched chain amino acids during intracellular replication of L. monocytogenes.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Comparative genomics of Listeria species.
- Record: found
- Abstract: found
- Article: not found
TCDB: the Transporter Classification Database for membrane transport protein analyses and information
- Record: found
- Abstract: found
- Article: not found