- Record: found
- Abstract: found
- Article: found
Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility
Read this article at
Abstract
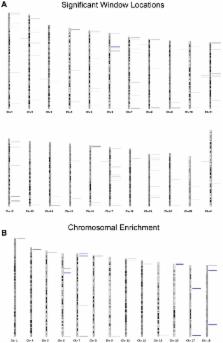
Recent evidence demonstrates a role for paternal aging on offspring disease susceptibility. It is well established that various neuropsychiatric disorders (schizophrenia, autism, etc.), trinucleotide expansion associated diseases (myotonic dystrophy, Huntington's, etc.) and even some forms of cancer have increased incidence in the offspring of older fathers. Despite strong epidemiological evidence that these alterations are more common in offspring sired by older fathers, in most cases the mechanisms that drive these processes are unclear. However, it is commonly believed that epigenetics, and specifically DNA methylation alterations, likely play a role. In this study we have investigated the impact of aging on DNA methylation in mature human sperm. Using a methylation array approach we evaluated changes to sperm DNA methylation patterns in 17 fertile donors by comparing the sperm methylome of 2 samples collected from each individual 9–19 years apart. With this design we have identified 139 regions that are significantly and consistently hypomethylated with age and 8 regions that are significantly hypermethylated with age. A representative subset of these alterations have been confirmed in an independent cohort. A total of 117 genes are associated with these regions of methylation alterations (promoter or gene body). Intriguingly, a portion of the age-related changes in sperm DNA methylation are located at genes previously associated with schizophrenia and bipolar disorder. While our data does not establish a causative relationship, it does raise the possibility that the age-associated methylation of the candidate genes that we observe in sperm might contribute to the increased incidence of neuropsychiatric and other disorders in the offspring of older males. However, further study is required to determine whether, and to what extent, a causative relationship exists.
Author Summary
There is a striking trend of delayed parenthood in developed countries due to secular and socioeconomic pressures. As a result, physicians commonly consult with concerned patients inquiring about the impact of advanced age on their ability to conceive healthy offspring. The concern has more frequently surrounded the effects of advanced maternal age, but recent evidence suggests negative effects of advanced paternal age as well. Specifically, studies have demonstrated increased incidence of neuropsychiatric and other disorders in the offspring of older males. In this study we have investigated a commonly hypothesized mechanism for this effect, namely sperm DNA methylation alteration. Our data indicate that specific genomic regions of DNA methylation are commonly altered with age, suggesting that some regions of the sperm genome are more susceptible than others to age-related epigenetic changes. Importantly, a significant portion of these alterations occur at genes known to be associated with schizophrenia and bipolar disorder, both of which display increased incidence in the offspring of older fathers. These data will be important in driving future studies aimed at determining the impact that these methylation alterations may have on offspring health and will thus enable couples at advanced reproductive ages to be more informed of possible risks.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
DNA methylation age of human tissues and cell types
- Record: found
- Abstract: found
- Article: not found
The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease.
- Record: found
- Abstract: found
- Article: not found