- Record: found
- Abstract: found
- Article: found
Altered Microbiota Contributes to Reduced Diet-Induced Obesity upon Cold Exposure
Read this article at
Summary
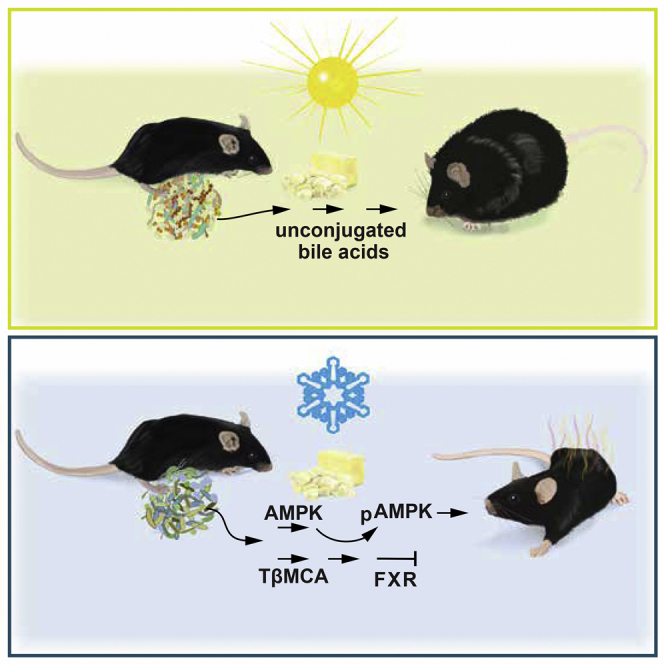
Maintenance of body temperature in cold-exposed animals requires induction of thermogenesis and management of fuel. Here, we demonstrated that reducing ambient temperature attenuated diet-induced obesity (DIO), which was associated with increased iBAT thermogenesis and a plasma bile acid profile similar to that of germ-free mice. We observed a marked shift in the microbiome composition at the phylum and family levels within 1 day of acute cold exposure and after 4 weeks at 12°C. Gut microbiota was characterized by increased levels of Adlercreutzia, Mogibacteriaceae, Ruminococcaceae, and Desulfovibrio and reduced levels of Bacilli, Erysipelotrichaceae, and the genus rc4-4. These genera have been associated with leanness and obesity, respectively. Germ-free mice fed a high-fat diet at room temperature gained less adiposity and improved glucose tolerance when transplanted with caecal microbiota of mice housed at 12°C compared to mice transplanted with microbiota from 29°C. Thus, a microbiota-liver-BAT axis may mediate protection against obesity at reduced temperature.
Graphical Abstract
Highlights
Abstract
Zietak et al. show that the gut microbiota contributes to the beneficial effects of cold on adiposity via a mechanism involving the production of conjugated bile acids and AMPK activation. Transfer of gut microbiota from cold-exposed mice reduces diet-induced obesity in germ-free recipient mice.
Related collections
Most cited references13
- Record: found
- Abstract: found
- Article: not found
Activated type 2 innate lymphoid cells regulate beige fat biogenesis.
- Record: found
- Abstract: found
- Article: not found
High-fat diet determines the composition of the murine gut microbiome independently of obesity.
- Record: found
- Abstract: found
- Article: not found