- Record: found
- Abstract: found
- Article: found
Conformational plasticity of RNA for target recognition as revealed by the 2.15 Å crystal structure of a human IgG–aptamer complex
Read this article at
Abstract
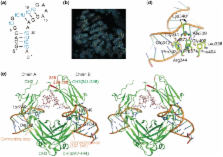
Aptamers are short single-stranded nucleic acids with high affinity to target molecules and are applicable to therapeutics and diagnostics. Regardless of an increasing number of reported aptamers, the structural basis of the interaction of RNA aptamer with proteins is poorly understood. Here, we determined the 2.15 Å crystal structure of the Fc fragment of human IgG1 (hFc1) complexed with an anti-Fc RNA aptamer. The aptamer adopts a characteristic structure fit to hFc1 that is stabilized by a calcium ion, and the binding activity of the aptamer can be controlled many times by calcium chelation and addition. Importantly, the aptamer–hFc1 interaction involves mainly van der Waals contacts and hydrogen bonds rather than electrostatic forces, in contrast to other known aptamer–protein complexes. Moreover, the aptamer–hFc1 interaction involves human IgG-specific amino acids, rendering the aptamer specific to human IgGs, and not crossreactive to other species IgGs. Hence, the aptamer is a potent alternative for protein A affinity purification of Fc-fusion proteins and therapeutic antibodies. These results demonstrate, from a structural viewpoint, that conformational plasticity and selectivity of an RNA aptamer is achieved by multiple interactions other than electrostatic forces, which is applicable to many protein targets of low or no affinity to nucleic acids.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase.
- Record: found
- Abstract: found
- Article: not found
Structure of the human telomerase RNA pseudoknot reveals conserved tertiary interactions essential for function.
- Record: found
- Abstract: found
- Article: not found