- Record: found
- Abstract: found
- Article: found
Necrotizing enterocolitis in premature infants—A defect in the brakes? Evidence from clinical and animal studies
Read this article at
Abstract
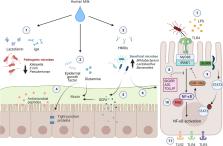
A key aspect of postnatal intestinal adaptation is the establishment of symbiotic relationships with co-evolved gut microbiota. Necrotizing enterocolitis (NEC) is the most severe disease arising from failure in postnatal gut adaptation in premature infants. Although pathological activation of intestinal Toll-like receptors (TLRs) is believed to underpin NEC pathogenesis, the mechanisms are incompletely understood. We postulate that unregulated aberrant TLR activation in NEC arises from a failure in intestinal-specific mechanisms that tamponade TLR signaling (the brakes). In this review, we discussed the human and animal studies that elucidate the developmental mechanisms inhibiting TLR signaling in the postnatal intestine (establishing the brakes). We then evaluate evidence from preclinical models and human studies that point to a defect in the inhibition of TLR signaling underlying NEC. Finally, we provided a framework for the assessment of NEC risk by screening for signatures of TLR signaling and for NEC prevention by TLR-targeted therapy in premature infants.
Related collections
Most cited references163
- Record: found
- Abstract: found
- Article: not found
MicroRNA therapeutics: towards a new era for the management of cancer and other diseases
- Record: found
- Abstract: found
- Article: not found
Pathogen recognition and innate immunity.
- Record: found
- Abstract: found
- Article: not found