- Record: found
- Abstract: found
- Article: found
The DNA Damage Response Pathway Contributes to the Stability of Chromosome III Derivatives Lacking Efficient Replicators
Read this article at
Abstract
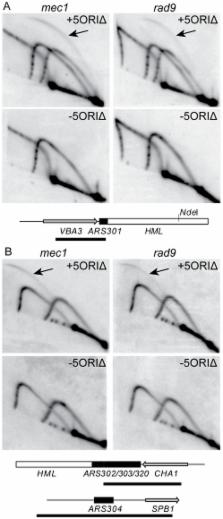
In eukaryotic chromosomes, DNA replication initiates at multiple origins. Large inter-origin gaps arise when several adjacent origins fail to fire. Little is known about how cells cope with this situation. We created a derivative of Saccharomyces cerevisiae chromosome III lacking all efficient origins, the 5ORIΔ-ΔR fragment, as a model for chromosomes with large inter-origin gaps. We used this construct in a modified synthetic genetic array screen to identify genes whose products facilitate replication of long inter-origin gaps. Genes identified are enriched in components of the DNA damage and replication stress signaling pathways. Mrc1p is activated by replication stress and mediates transduction of the replication stress signal to downstream proteins; however, the response-defective mrc1 AQ allele did not affect 5ORIΔ-ΔR fragment maintenance, indicating that this pathway does not contribute to its stability. Deletions of genes encoding the DNA-damage-specific mediator, Rad9p, and several components shared between the two signaling pathways preferentially destabilized the 5ORIΔ-ΔR fragment, implicating the DNA damage response pathway in its maintenance. We found unexpected differences between contributions of components of the DNA damage response pathway to maintenance of ORIΔ chromosome derivatives and their contributions to DNA repair. Of the effector kinases encoded by RAD53 and CHK1, Chk1p appears to be more important in wild-type cells for reducing chromosomal instability caused by origin depletion, while Rad53p becomes important in the absence of Chk1p. In contrast, RAD53 plays a more important role than CHK1 in cell survival and replication fork stability following treatment with DNA damaging agents and hydroxyurea. Maintenance of ORIΔ chromosomes does not depend on homologous recombination. These observations suggest that a DNA-damage-independent mechanism enhances ORIΔ chromosome stability. Thus, components of the DNA damage response pathway contribute to genome stability, not simply by detecting and responding to DNA template damage, but also by facilitating replication of large inter-origin gaps.
Author Summary
Loss of genome integrity underlies aspects of aging and human disease. During DNA replication, two parallel signaling pathways play important roles in the maintenance of genome integrity. One pathway detects DNA damage, while the other senses replication stress. Both pathways activate responses that include arrest of cell cycle progression, giving cells time to cope with the problem. These pathways have been defined by treating cells with compounds that induce either replication stress or DNA damage, but little is known about their roles during unperturbed DNA replication. They may be important when several adjacent replication origins fail to initiate and forks from flanking origins must replicate longer regions of DNA than normal to complete replication. We have used a derivative of budding yeast chromosome III lacking all efficient replication origins to identify mutants that preferentially destabilize this chromosome fragment, which mimics a chromosome with a large inter-origin gap. We found that the DNA damage response pathway, but not the replication stress response pathway, plays an important role in maintaining this fragment. The signal recognized in this case may be replisome failure rather than forks stalled at endogenous DNA damage.
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae.
- Record: found
- Abstract: found
- Article: not found
New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae.
- Record: found
- Abstract: found
- Article: not found