- Record: found
- Abstract: found
- Article: found
A novel acetyltransferase p300 inhibitor ameliorates hypertension-associated cardio-renal fibrosis
Read this article at
ABSTRACT
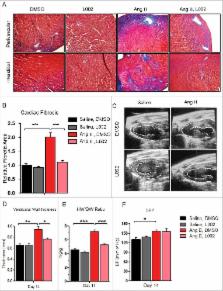
Hypertension-associated end-organ damage commonly leads to cardiac and renal fibrosis. As no effective anti-fibrotic therapy currently exists, the unchecked progression of fibrogenesis manifests as cardio-renal failure and early death. We have previously shown that FATp300—p300 with intrinsic factor acetyltransferase activity—is an essential epigenetic regulator of fibrogenesis, and is elevated in several fibrotic tissues. In this report, we investigate the therapeutic efficacy of a novel FATp300 inhibitor, L002, in a murine model of hypertensive cardio-renal fibrosis. Additionally, we examine the effects of L002 on cellular pro-fibrogenic processes and provide mechanistic insights into its antifibrogenic action. Utilizing cardiac fibroblasts, podocytes, and mesangial cells, we demonstrate that L002 blunts FATp300-mediated acetylation of specific histones. Further, incubating cells with L002 suppresses several pro-fibrogenic processes including cellular proliferation, migration, myofibroblast differentiation and collagen synthesis. Importantly, systemic administration of L002 in mice reduces hypertension-associated pathological hypertrophy, cardiac fibrosis and renal fibrosis. The anti-hypertrophic and anti-fibrotic effects of L002 were independent of blood pressure regulation. Our work solidifies the role of epigenetic regulator FATp300 in fibrogenesis and establishes it as a pharmacological target for reducing pathological matrix remodeling and associated pathologies. Additionally, we discover a new therapeutic role of L002, as it ameliorates hypertension-induced cardio-renal fibrosis and antagonizes pro-fibrogenic responses in fibroblasts, podocytes and mesangial cells.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases.
- Record: found
- Abstract: found
- Article: not found
Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300.
- Record: found
- Abstract: found
- Article: not found