- Record: found
- Abstract: found
- Article: found
The STAR protein QKI-7 recruits PAPD4 to regulate post-transcriptional polyadenylation of target mRNAs
Read this article at
Abstract
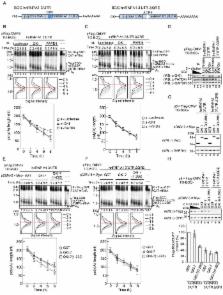
Emerging evidence has demonstrated that regulating the length of the poly(A) tail on an mRNA is an efficient means of controlling gene expression at the post-transcriptional level. In early development, transcription is silenced and gene expression is primarily regulated by cytoplasmic polyadenylation. In somatic cells, considerable progress has been made toward understanding the mechanisms of negative regulation by deadenylation. However, positive regulation through elongation of the poly(A) tail has not been widely studied due to the difficulty in distinguishing whether any observed increase in length is due to the synthesis of new mRNA, reduced deadenylation or cytoplasmic polyadenylation. Here, we overcame this barrier by developing a method for transcriptional pulse-chase analysis under conditions where deadenylases are suppressed. This strategy was used to show that a member of the Star family of RNA binding proteins, QKI, promotes polyadenylation when tethered to a reporter mRNA. Although multiple RNA binding proteins have been implicated in cytoplasmic polyadenylation during early development, previously only CPEB was known to function in this capacity in somatic cells. Importantly, we show that only the cytoplasmic isoform QKI-7 promotes poly(A) tail extension, and that it does so by recruiting the non-canonical poly(A) polymerase PAPD4 through its unique carboxyl-terminal region. We further show that QKI-7 specifically promotes polyadenylation and translation of three natural target mRNAs (hnRNPA1, p27 kip1 and β-catenin) in a manner that is dependent on the QKI response element. An anti-mitogenic signal that induces cell cycle arrest at G1 phase elicits polyadenylation and translation of p27 kip1 mRNA via QKI and PAPD4. Taken together, our findings provide significant new insight into a general mechanism for positive regulation of gene expression by post-transcriptional polyadenylation in somatic cells.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Ending the message: poly(A) signals then and now.
- Record: found
- Abstract: found
- Article: not found
Circularization of mRNA by eukaryotic translation initiation factors.
- Record: found
- Abstract: found
- Article: not found