- Record: found
- Abstract: found
- Article: found
Drosophila IAP antagonists form multimeric complexes to promote cell death
Read this article at
Abstract
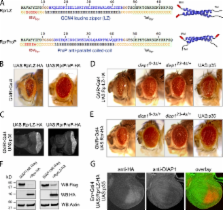
Self- and hetero-association of the pro-apoptotic proteins Reaper, Hid, and Grim is required for efficient induction of the cell death program.
Abstract
Apoptosis is a specific form of cell death that is important for normal development and tissue homeostasis. Caspases are critical executioners of apoptosis, and living cells prevent their inappropriate activation through inhibitor of apoptosis proteins (IAPs). In Drosophila, caspase activation depends on the IAP antagonists, Reaper (Rpr), Head involution defective (Hid), and Grim. These proteins share a common motif to bind Drosophila IAP1 (DIAP1) and have partially redundant functions. We now show that IAP antagonists physically interact with each other. Rpr is able to self-associate and also binds to Hid and Grim. We have defined the domain involved in self-association and demonstrate that it is critical for cell-killing activity in vivo. In addition, we show that Rpr requires Hid for recruitment to the mitochondrial membrane and for efficient induction of cell death in vivo. Both targeting of Rpr to mitochondria and forced dimerization strongly promotes apoptosis. Our results reveal the functional importance of a previously unrecognized multimeric IAP antagonist complex for the induction of apoptosis.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31.
- Record: found
- Abstract: found
- Article: not found
Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins.
- Record: found
- Abstract: found
- Article: not found