- Record: found
- Abstract: found
- Article: not found
The Level of the Transcription Factor Pax6 Is Essential for Controlling the Balance between Neural Stem Cell Self-Renewal and Neurogenesis
Read this article at
Abstract
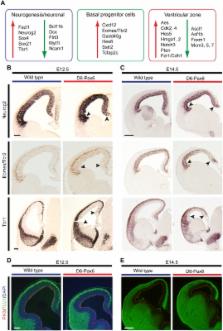
Neural stem cell self-renewal, neurogenesis, and cell fate determination are processes that control the generation of specific classes of neurons at the correct place and time. The transcription factor Pax6 is essential for neural stem cell proliferation, multipotency, and neurogenesis in many regions of the central nervous system, including the cerebral cortex. We used Pax6 as an entry point to define the cellular networks controlling neural stem cell self-renewal and neurogenesis in stem cells of the developing mouse cerebral cortex. We identified the genomic binding locations of Pax6 in neocortical stem cells during normal development and ascertained the functional significance of genes that we found to be regulated by Pax6, finding that Pax6 positively and directly regulates cohorts of genes that promote neural stem cell self-renewal, basal progenitor cell genesis, and neurogenesis. Notably, we defined a core network regulating neocortical stem cell decision-making in which Pax6 interacts with three other regulators of neurogenesis, Neurog2, Ascl1, and Hes1. Analyses of the biological function of Pax6 in neural stem cells through phenotypic analyses of Pax6 gain- and loss-of-function mutant cortices demonstrated that the Pax6-regulated networks operating in neural stem cells are highly dosage sensitive. Increasing Pax6 levels drives the system towards neurogenesis and basal progenitor cell genesis by increasing expression of a cohort of basal progenitor cell determinants, including the key transcription factor Eomes/Tbr2, and thus towards neurogenesis at the expense of self-renewal. Removing Pax6 reduces cortical stem cell self-renewal by decreasing expression of key cell cycle regulators, resulting in excess early neurogenesis. We find that the relative levels of Pax6, Hes1, and Neurog2 are key determinants of a dynamic network that controls whether neural stem cells self-renew, generate cortical neurons, or generate basal progenitor cells, a mechanism that has marked parallels with the transcriptional control of embryonic stem cell self-renewal.
Author Summary
Neural stem cells make all of the neurons in the brain. A key feature of these cells is the ability to regulate the balance between making more neural stem cells, the process of self-renewal, and making nerve cells, the process of neurogenesis. Too much self-renewal would result in a brain with too few neurons and abnormal circuitry; too much neurogenesis would deplete all of the neural stem cells too quickly, resulting in a small brain and neurological abnormalities. Little is currently known of the how neural stem cells control this fundamental choice. We used one transcription factor, Pax6, which is important for this decision, as an entry point to define the cellular networks controlling neural stem cell self-renewal and neurogenesis in the developing mouse brain. We found that the relative amount of Pax6 controls the balance between self-renewal and neurogenesis in neural stem cells. Increasing Pax6 levels drives the system towards neurogenesis, at the expense of self-renewal, by turning on a genetic programme for making neurons, whereas decreasing Pax6 turns off the genetic programme for neural stem cell self-renewal. In both cases, altering the levels of Pax6 ultimately leads to a small brain, but through very different mechanisms.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells.
- Record: found
- Abstract: found
- Article: not found
P53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation
- Record: found
- Abstract: found
- Article: not found
