- Record: found
- Abstract: found
- Article: found
Connexin 37 Regulates the Kv1.3 Pathway and Promotes the Development of Atherosclerosis
Read this article at
Abstract
Objective
To investigate the mechanism of Connexin 37 (Cx37) and Kv1.3 pathways in atherosclerosis (AS).
Methods
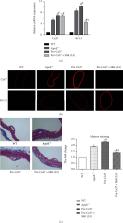
ApoE −/− mice were given a high-fat diet to establish atherosclerosis (AS) model, and macrophages in mice were isolated and extracted to transfect Cx37 vectors with silencing or overexpressing, and Kv1.3 pathway blockers were used to inhibit the pathway activity. The indexes of body weight, blood glucose, and blood lipid of mice were collected. The protein and mRNA expression levels of Cx37 and Kv1.3 were detected by reverse transcription-PCR (RT-PCR), Western blot, and immunofluorescence technique. Oil red O staining was used to observe plaque area. Masson staining was used to detect collagen content. The concentrations of chemokine CCL7 were quantified using the ELISA kits. CCK8 was used to detect cell proliferation.
Results
Cx37 and Kv1.3 were highly expressed in macrophages of AS mice, and the expression of Kv1.3 and CCL7 decreased after Cx37 was silenced, and the proliferation of macrophages was also decreased. Wild-type mice and AS model mice were treated with Cx37 overexpression vectors and Kv1.3 pathway blocking, and it was found that Cx37 overexpression could improve the blood lipid and blood glucose levels and increase the area of AS in AS mice. However, blocking the activity of Kv1.3 pathway can reduce the levels of blood lipid and blood glucose, increase the body weight of mice, and reduce the area of AS mice. Blocking the activity of Kv1.3 pathway can slow down the plaque development of AS mice and make its indexes close to wild-type mice. And the use of Kv1.3 pathway blockers on the basis of overexpression of Cx37 indicated that inhibition of Kv1.3 pathway activity did not affect the expression of Cx37, but could inhibit the collagen content in the plaque area of AS mice, inhibit the expression of chemokine CCL7, and reverse the effect of Cx37 overexpression.
Related collections
Most cited references27

- Record: found
- Abstract: found
- Article: not found
The immune system in atherosclerosis.
- Record: found
- Abstract: found
- Article: not found