- Record: found
- Abstract: found
- Article: found
New insights into small‐cell lung cancer development and therapy
Read this article at
Abstract
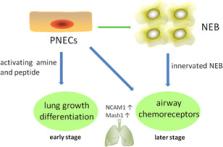
Small‐cell lung cancer (SCLC) accounts for approximately 15% of lung cancer cases; however, it is characterized by easy relapse and low survival rate, leading to one of the most intractable diseases in clinical practice. Despite decades of basic and clinical research, little progress has been made in the management of SCLC. The current standard first‐line regimens of SCLC still remain to be cisplatin or carboplatin combined with etoposide, and the adverse events of chemotherapy are by no means negligible. Besides, the immunotherapy on SCLC is still in an early stage and novel studies are urgently needed. In this review, we describe SCLC development and current therapy, aiming at providing useful advices on basic research and clinical strategy.
Related collections
Most cited references104

- Record: found
- Abstract: found
- Article: found
Management of acquired resistance to EGFR TKI–targeted therapy in advanced non-small cell lung cancer
- Record: found
- Abstract: found
- Article: not found
Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group.
- Record: found
- Abstract: found
- Article: not found